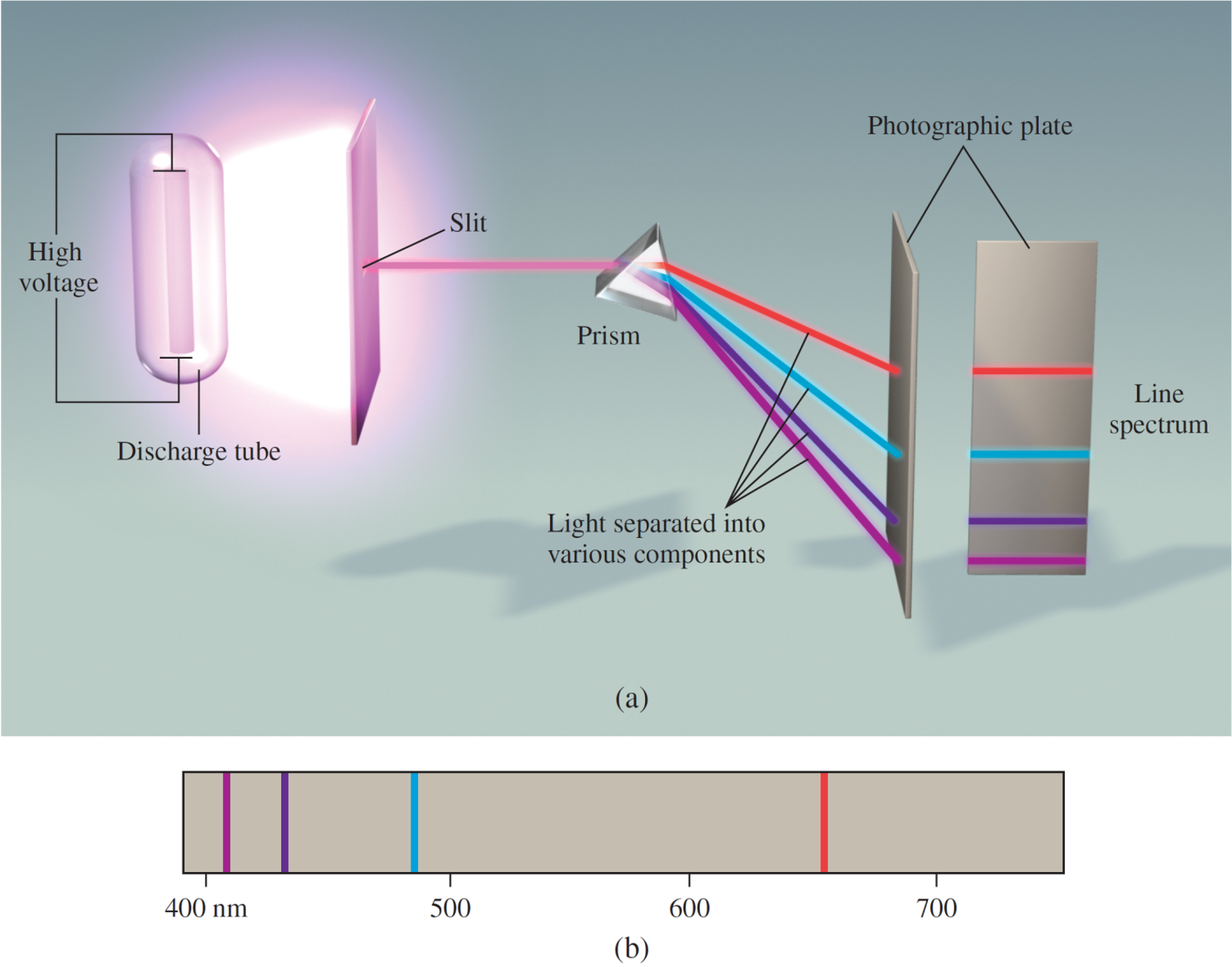
The emission spectrum of a substance can be seen by energizing a sample of material with some form of energy. The “red hot” or “white hot” glow of an iron bar removed from a fire is the visible portion of its emission spectrum. The emission spectrum of both sunlight and a heated solid are continuous; all wavelengths of visible light are present. Line spectra are the emission of light only at specific wavelengths. Every element has its own unique emission spectrum.

Figure 3.1: Line spectra of hydrogen.
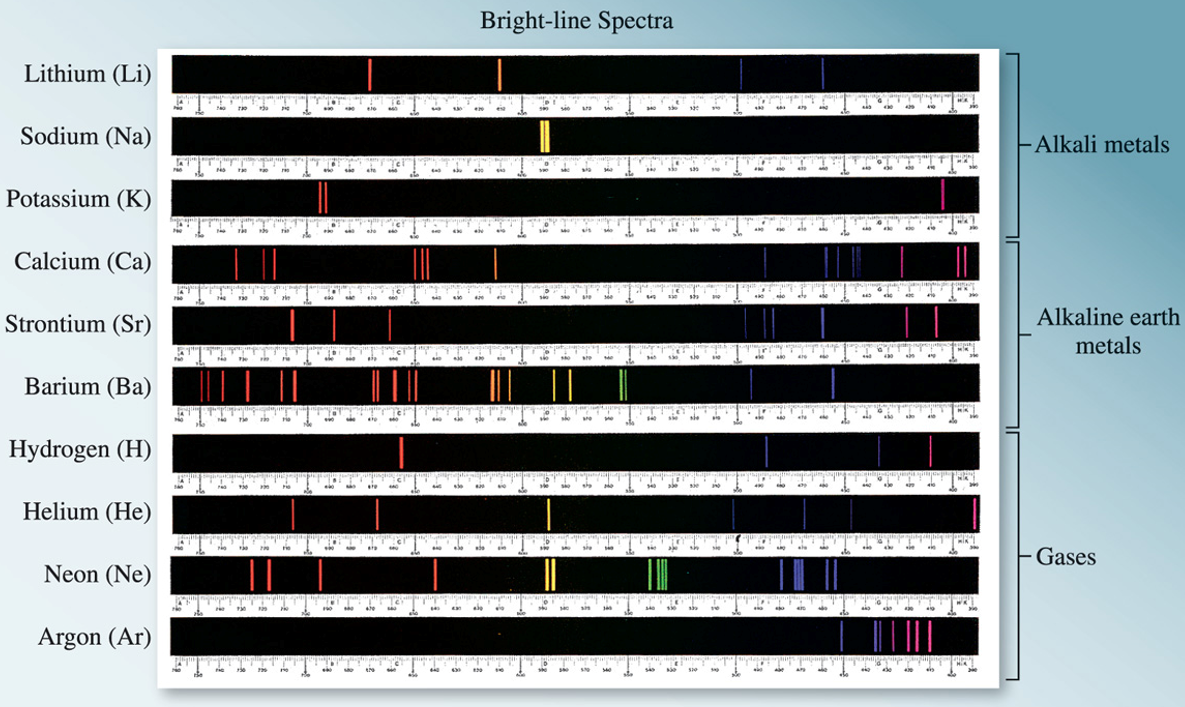 Line spectra for various elements." width="100%" />
Line spectra for various elements." width="100%" />
Figure 3.2: Line spectra for various elements.
The Rydberg equation can be used to calculate the wavelengths of the four visible lines in the emission spectrum of hydrogen
where R∞ is the Rydberg constant (R∞ = 1.09737317×10 7 m –1 ), λ is the wavelength of the observed line in the hydrogen emission spectrum, n1 and n2 are positive integers (where n2 > n1).
The associated energy change (ΔE) with respect to the observed wavelength can be expressed as
\[\Delta E = -2.18\times 10^~\mathrm \left ( \dfrac - \dfrac \right ) \]
where ni is the initial state and nf is the final state. If the energy change is negative, the final state has lower energy than the initial state (energy was lost/emitted; relaxation). If the energy change is positive, the the final state has higher energy than the initial state (energy was absorbed; excitation).
Bohr’s theory explains the line spectrum of the hydrogen atom. Radiant energy absorbed by the atom causes the electron to move from the ground state (n = 1) to an excited state (n > 1). Conversely, radiant energy is emitted when the electron moves from a higher–energy state to a lower–energy excited state or the ground state.
The quantized movement of the electron from one energy state to another is analogous to a ball moving and down steps.
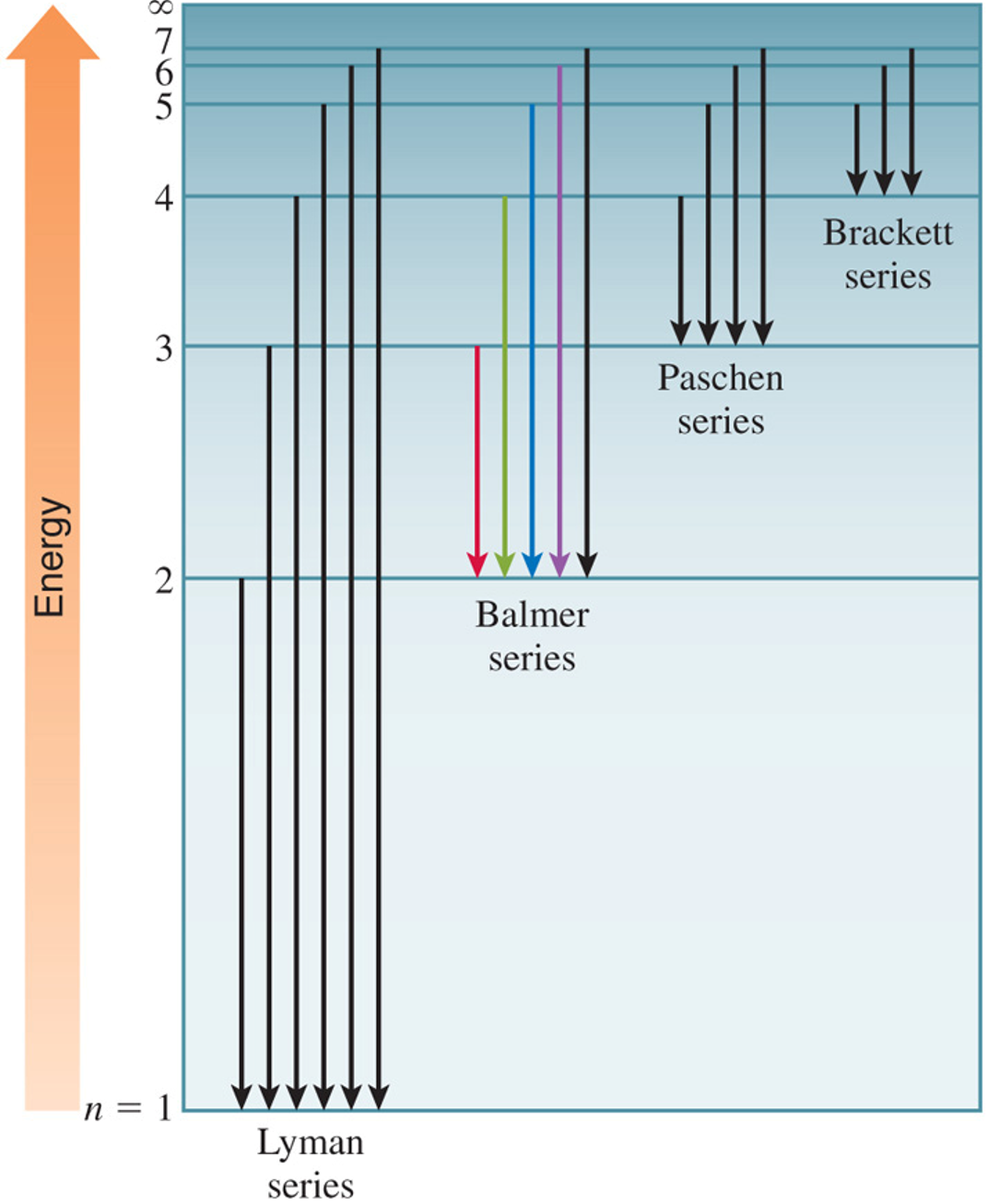
Neils Bohr attributed the emission of radiation by an energized hydrogen atom to the electron dropping from a higher–energy orbit to a lower one. As the electron dropped, it gave up a quantum of energy in the form of light. Bohr showed that the energies of the electron in a hydrogen atom are given by the equation:
where n is a positive integer. As an electron gets closer to the nucleus, n decreases. En becomes larger in absolute value (more negative) as n gets smaller. En is most negative when n = 1 (called the ground state, the lowest energy state of the atom). The stability of the electron decreases as n increases. Each energy state in which n > 1 is called an excited state.